Return list
Return list
Huaxin Medical pressed the acceleration button and obtained 10 registration certificates in the first half of this year!
With the evolution of endoscopic consumables into a development trend in the domestic market, disposable endoscopes, which have three major advantages of reducing risks, costs, and improving surgical efficiency, will become one of the important tracks in the emerging endoscopic market.

As one of the leading enterprises in disposable endoscopes, Huaxin Medical has accelerated its development in the whole industry of disposable endoscopes. Since January, its endoscopic products have frequently obtained certificates, with a total of 10 at present, including 7 registration certificates for disposable endoscopes. Currently, Huaxin Medical has not only obtained domestic medical device certifications, but also certifications from the United States, the European Union, Australia, Argentina, Canada, and other countries.

Creating a new ecosystem for disposable endoscopes
In 2009, disposable electronic endoscopes emerged. Around 2010, companies focused on disposable endoscopes emerged in China, and by 2020, they had experienced a collective outbreak.
Huaxin Medical was founded in 2018 and emerged in the second stage of developing disposable endoscopes in China. Subsequently, it focused on the research and development, production, and sales of disposable endoscopes. Shortly after its establishment, Huaxin Medical's disposable electronic bronchoscope obtained registration certificates for domestic NMPA, EU CE, US FDA, and Japan PMDA.

It is understood that the disposable electronic bronchoscope has an extremely smooth front-end control of the endoscope body, no empty segment operation, stable torque, and can achieve 210 ° bending up and down, with 1:1 synchronous transmission, making clinical operations more flexible and reliable. Moreover, it has 6 instrument channels that can accommodate various sizes of interventional therapy consumables, thereby meeting the diverse clinical needs.
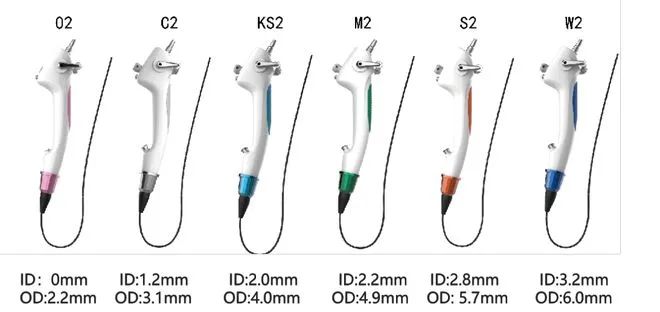
A study conducted by UCSD pointed out that Huaxin Medical's disposable electronic bronchoscope is comparable to reusable endoscopes in terms of overall usage evaluation, product quality, usability, operability, image quality, and other aspects, making it a leader in the industry.
With the mature technical strength of the research and development team and the deep cooperation with Olympus, the disposable electronic bronchoscope product has been recognized by the market. It has not only created a new ecosystem for domestic disposable endoscopes, met clinical needs, and become a powerful helper for doctors, but also been recognized by the foreign market. It has been launched in more than 30 countries and regions, including China, the United States, the European Union, and Japan.
Press the accelerator button for the layout of disposable endoscopes
According to market data, Huaxin Medical has currently publicly applied for 273 patents, 204 valid patents, and 17 international patents. Among them, utility models account for 58.6%, and invention announcements account for 25.27%.
From the data, it can be seen that the company has been very active in innovating its endoscope products recently.
As of now, Huaxin Medical has developed world leading projects such as disposable electronic bronchoscopy, disposable electronic ureteroscopy, disposable electronic bladder pyeloscopy, and disposable electronic nasopharyngeal endoscopy, and has successively applied for product registration certificates.

In early January 2023, Huaxin Medical's disposable electronic rhinolaryngoscope obtained the NMPA registration certificate; on March 10th, the medical endoscope image processor was awarded the EU MDR certificate; on April 25th, the disposable electronic bronchoscope successfully obtained the Australian TGA certificate; on April 27th, the medical endoscope image processor successfully obtained the Argentine A.N.M.A.T. registration certificate; on May 5th, the disposable electronic bronchoscope successfully obtained the Canadian HC registration certificate; on May 10th, the disposable electronic rhinolaryngoscope and the disposable electronic cystoscope-nephroscope successfully obtained the Australian TGA registration certificates; on May 12th, the medical endoscope image processor successfully obtained the Australian TGA registration certificate; on May 18th, the medical endoscope image processor was approved by the US FDA; and on May 19th, the disposable electronic ureteroscope-nephroscope was approved by the US FDA.
From this, it can be seen that as a leading enterprise in disposable endoscopes, Huaxin Medical not only depicts a product map, but also further expands its sales map, realizes market linkage at home and abroad, and promotes the widespread popularity of disposable endoscopes in domestic and foreign markets.








 Return list
Return list